Paule Bellwood, Ph.D. • Jeffrey J. Stegman, B.Sc. • Gary E. Schwartz, Ph.D.
Keywords
Electromagnetic Field • EMF • Electromagnetic Field Mitigation • EMF Mitigation • Focused Life-Force Energy • FLFE • Sensitivity to Electromagnetic Field • Sensitivity to EMF • Electromagnetic Hypersensitivity
Abstract
Sensitivities to Electromagnetic Fields (EMFs) can affect individuals to varying degrees and one of the main strategies for relieving the symptoms caused by these fields is limiting exposure to them. Focused Life-Force Energy (FLFE) has developed an EMF mitigating service that is aimed at providing individuals relief from EMFs without having to avoid them or without having to limit one’s exposure to them. This study explored the effects of this service on 40 volunteers over a 45-day intervention period. The results showed reductions in all 30 symptoms tested and the biggest relief was observed both for most severe symptoms and in the most sensitive subgroup.
Introduction
Sensitivities to Electromagnetic Fields (EMFs), also known as Electromagnetic Hypersensitivity (EHS) or “Microwave syndrome”, are a clinical condition that encompasses various nervous system and skin symptoms as well as other health problems ranging from issues with neurological and cognitive functioning to negative autonomic nervous system effects [1, 2]. Typically, these symptoms and effects occur after exposure to EMFs in the environment [2]. EHS symptoms can be similar to those frequently reported by individuals with multiple chemical sensitivities (MCSs) [2].
Some researchers call for limiting exposure to EMFs [2], but new technologies are being developed and implemented at an increasing pace, making the exposure to EMFs practically unavoidable. Others are suggesting the need for EMF mitigating approaches to alleviate the effects of exposure to EMFs [3].
Focused Life-Force Energy (FLFE) has developed an EMF mitigation service that is aimed at alleviating the symptoms of EMF sensitivities (https://www.flfe.net/). Its effects and mechanisms of action are not yet fully understood. This research study aims to continue scientific exploration into these effects, thus intending to validate and extend many of the positive and mostly spontaneous reports (i.e., anecdotal evidence) from its past and current FLFE customers.
Background
EHS is a syndrome that often presents with common complaints (e.g., fatigue, headaches, general weakness, ringing in the ears, insomnia, brain fog and difficulty concentrating, irritability, aches and pains, difficulty with balance and vertigo, and even neuropsychiatric effects) that can also be attributed to other syndromes and conditions (e.g., chronic fatigue, fibromyalgia, multiple chemical sensitivity, depression, etc. [1, 4, 5]. In addition, the adverse health effects of EMFs are “a contentious issue [and] […] primary care physicians have no objective diagnostic algorithms by which to diagnose EHS”, thus often resulting in EHS sufferers being “referred to a psychiatrist” [4, p. 217].
There is some evidence to suggest “that these symptoms are triggered by exposure to EMFs in sensitive individuals”, including “both the extra low electromagnetic fields (ELFs) coming from electricity and the radiofrequency (RF) EMFs coming from radar, communication devices, Wi-Fi, smart meters and many other forms of wireless devices” [4, p.217].
Currently, one of the main recommendations to avoid EHS is limiting exposure to EMFs [2]; however, the EMFs and RFs seem to be ever more prevalent in our environments, in some cases increasing between 20.1 and 57.1% annually [6] thus making electromagnetic radiation inescapable [2]. Others are calling for developing and implementing ways to mitigate EMFs in the environment, such as setting a specific threshold for the amount of power radiated per unit volume at a distance [3] or by using reconfigurable intelligent surfaces to manipulate the electromagnetic environment [7].
FLFE is a Canadian company offering a consciousness-raising subscription-based service for a property or around an object. The FLFE system is designed to focus available life-force energy and to activate a high consciousness field at a specified location (i.e., legal address or geographic coordinates) or around a personal object (i.e., mobile phone). The higher-level consciousness field, in combination with other enhancements, is intended to increase the beneficial nature of the local environment. The FLFE service claims are extraordinary [8] in terms of mainstream science and various experiments, such as the one detailed in this paper, have been conducted to explore the effects of the purported beneficial environmental changes. FLFE’s experimental philosophy is to first explore the effects (i.e., ‘Is something happening?’) and then, when possible and practical, explore the mechanisms of action. For more information, please see the FLFE Gold Standard research statement (https://www.flfe.net/research).
Recently, a pilot study (n=10) was conducted, and the results of this study are available here: https://www.flfe.net/emf-data/. The replication of the pilot study was intended as a way to confirm the findings in the pilot with a larger number of participants thus justifying the next phase of this research, discussed in the Conclusion and Future Directions section.
Methods
The survey instrument from the pilot study was used again for the replication study. The instrument was developed in collaboration with Dr. Tania M. Slawecki from the Materials Research Institute, the Pennsylvania State University. It includes questions on 30 symptoms, focusing on their intensities and frequencies and their rankings from 0 to 10.
For the intensities:
- 0 (zero) indicated that the individual did NOT have the symptom.
- 1 indicated a mild or minor discomfort from the symptom.
- 5 indicated a moderately uncomfortable symptom – tolerable but just barely.
- 10 indicated severe discomfort from the symptom – an extremely uncomfortable, painful, and/or debilitating symptom, or potentially life-threatening.
For the frequencies:
- 0 (zero) indicated that the individual did NOT experience the symptom.
- 1 indicated that the individual experienced the symptom infrequently, 10% of the time or less which works out to be about 3 times a month or less.
- 2.5 indicated that the individual experienced the symptom about 25% of the time, which works out to be 1-2 times per week.
- 5 indicated that the individual experienced the symptom about 50% of the time, say, 3-4 times per week.
- 10 indicated that the individual experienced the symptom 100% of the time, every hour of every day (continuous or chronic).
The initial (i.e., baseline) survey also included questions on adverse effects from 18 common household devices (e.g., energy-saving lightbulbs) and structures in the environment (e.g., cellphone towers). The Baseline survey instrument is available in Appendix A.
Participants were recruited via FLFE’s website, through FLFE’s Facebook group, and by advertising the study in podcasts and interviews (e.g., Coast to Coast).
A total of three surveys were administered to each study participant over a period of 45 days. The surveys were administered remotely using the Smartsheet online software (for more information, please visit https://www.smartsheet.com/). The recruitment was recurring and, therefore, the survey administration was continuous until a desired number of participants was reached (i.e., rather than recruiting all participants as a group and advancing them through the study as a cohort, each participant went through their own study timeline lasting 45 days).
For each participant, the Baseline survey was administered at Day 0, after which the intervention was applied (i.e., the FLFE’s EMF mitigation service was turned on). The Smarter EMF version of the service was used for the duration of the study. The Follow-up survey was administered at Day 15 and the Final survey was administered at Day 45. Participants received six months of complimentary FLFE Flagship service on their properties and phones following the study period. The Flagship service includes additional programs and features.
Results
38 females and 2 males participated in the replication study. Participants’ ages ranged from 27 to 79 years old (mean for the age was 54.3 years old). For analysis purposes, the intensity and frequency ratings were combined to create symptom scores (i.e., average of intensity and frequency ratings, per symptom). 36 out of 40 participants (i.e., 90%) reported reductions in their symptom scores following the 45-day intervention (Mean reduction=-1.39, t=6.769, df=39, p<0.0000001 Figure 1).
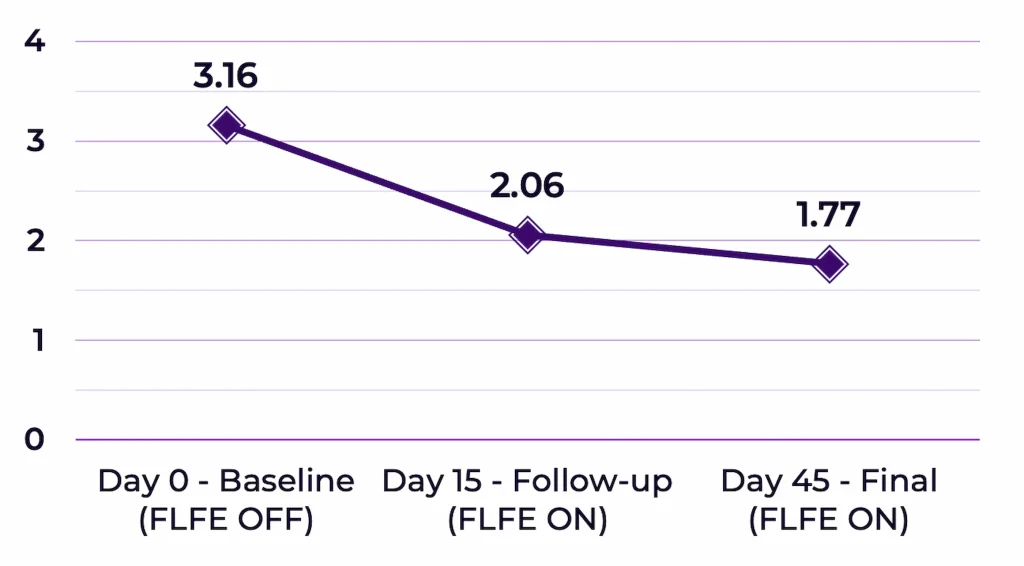
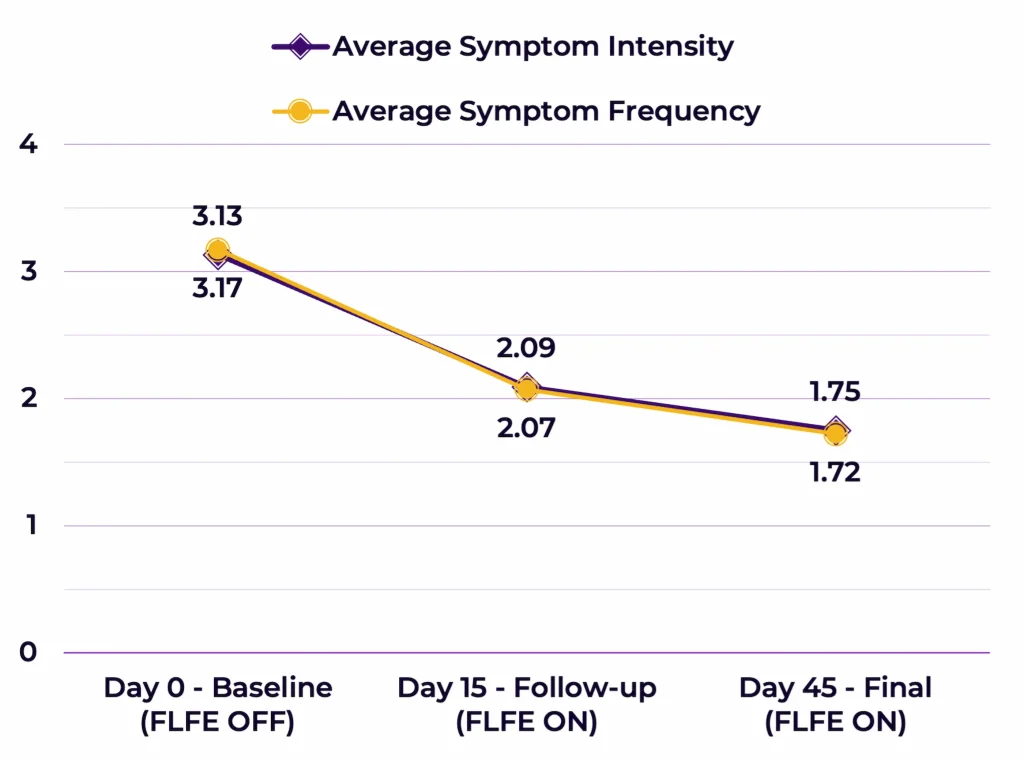
Analyses of variance revealed that the overall averaged symptoms intensity effect was F(2,78)=36.259, p<0.000005 and the overall averaged symptoms frequency effect was F(2,78)=11.005, p<0.0005; see Figure 2).
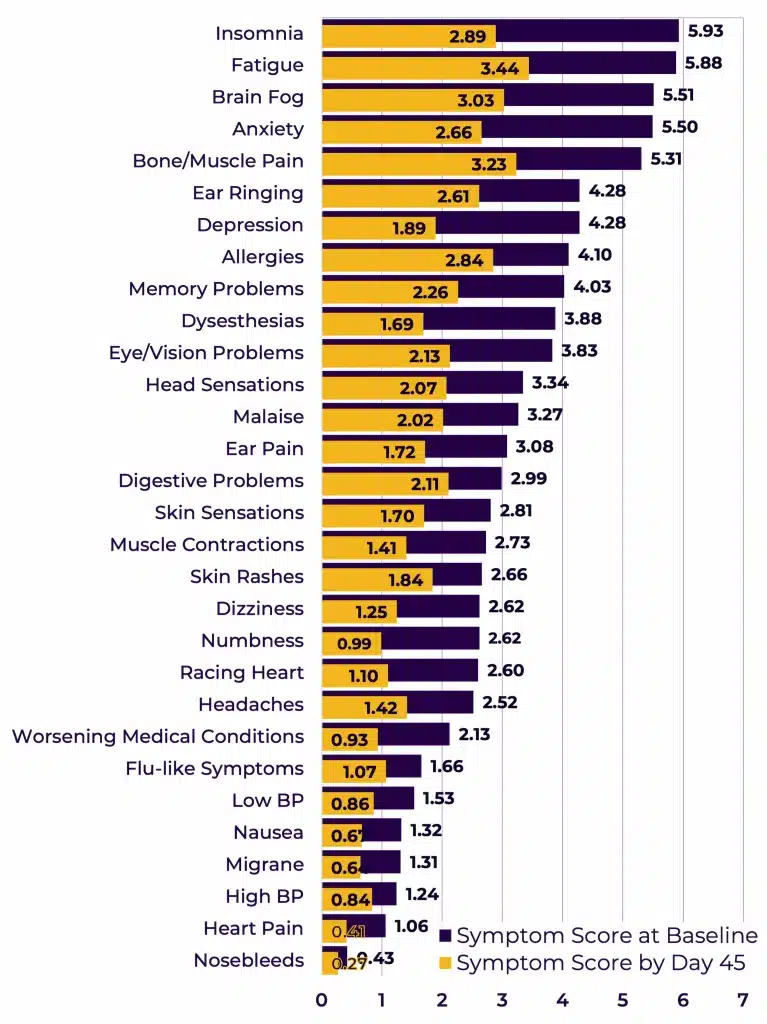
All symptom scores at Day 0 and their reductions by Day 45 were assessed by each individual symptom, out of 30 (Figure 3).
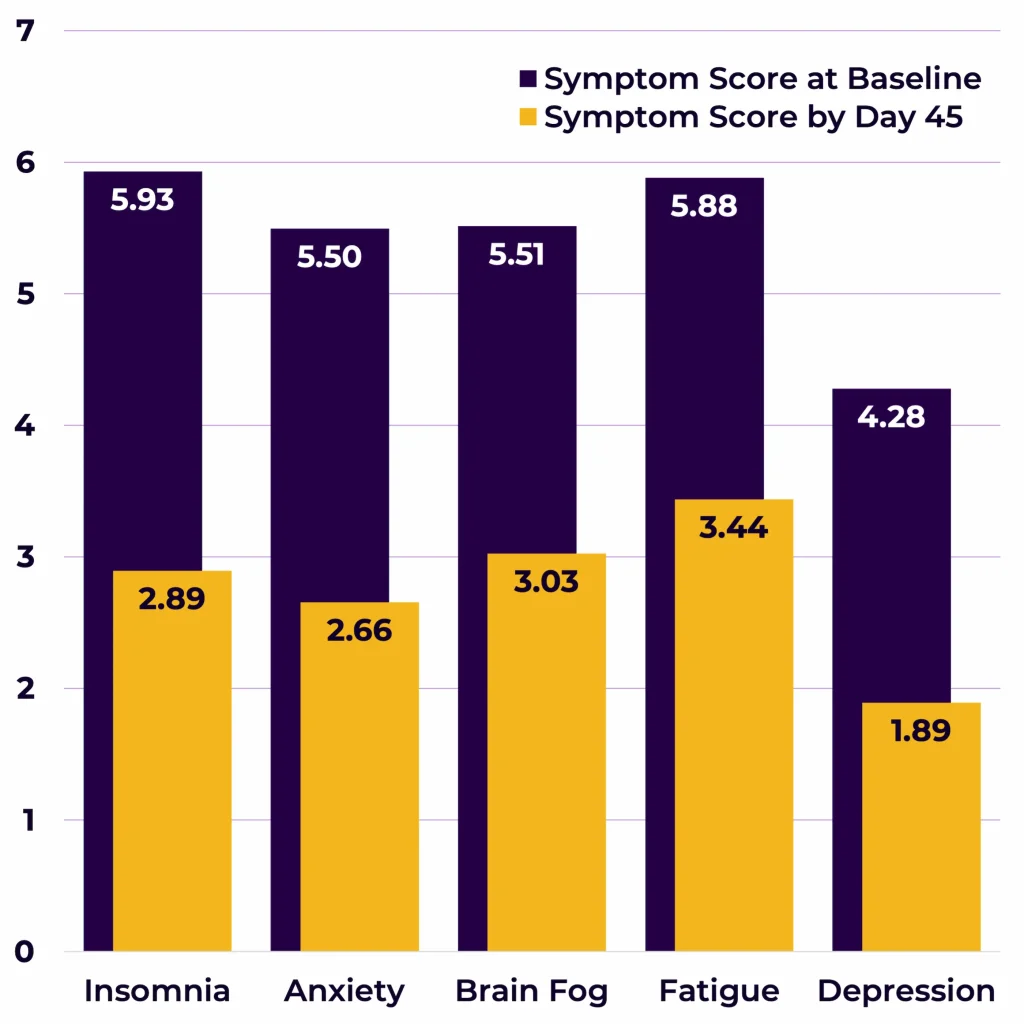
Five largest EMF mitigation effects were observed for the following symptoms (Figure 4):
- Insomnia (-3.04)
- Anxiety (-2.84)
- Brain Fog (-2.49)
- Fatigue (-2.44)
- Depression (-2.38).
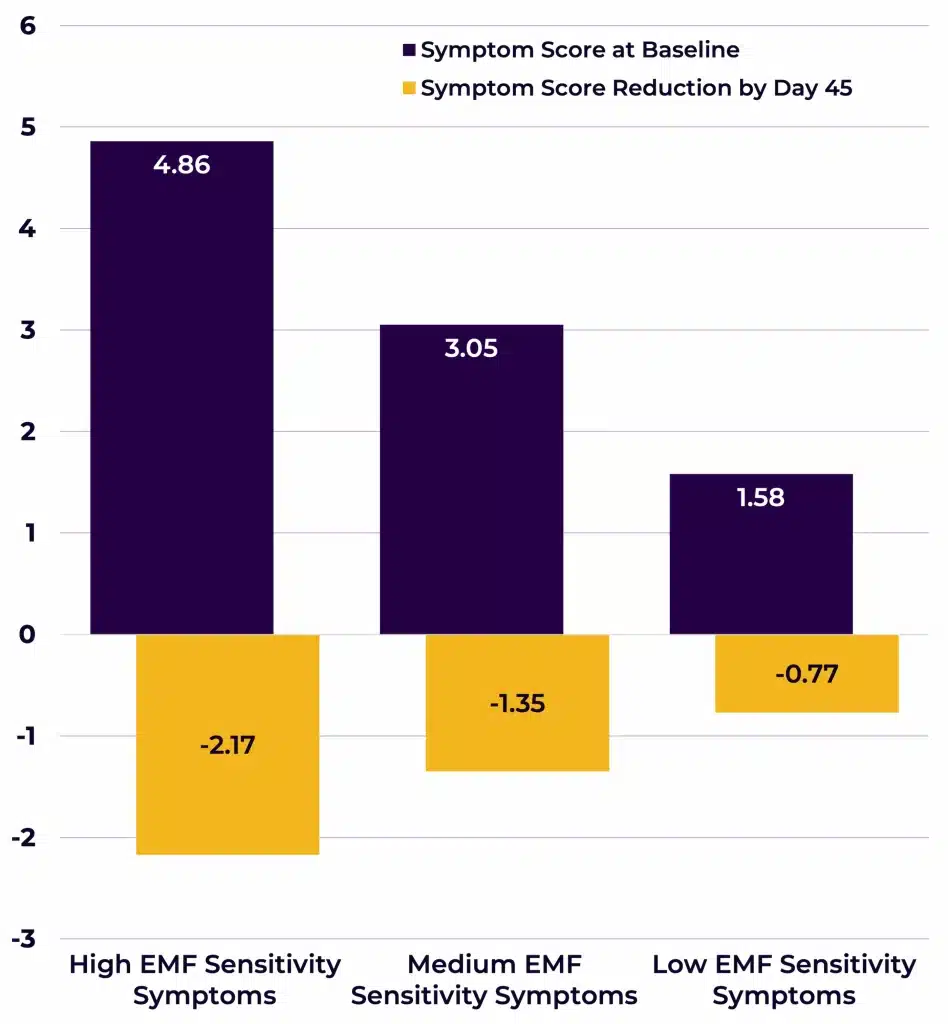
Symptom scores were also ranked by severity (i.e., split into “High”, “Medium”, and “Low” subsets of symptom severity, Figure 5). Symptom decreases were observed in all three symptom score subsets, but the biggest symptom decrease (-2.17) was observed in the “High” symptom score subset (i.e., the EMF mitigation had the strongest effects on the worst symptoms; F(2,37) = 21.265, p < 0.000005).
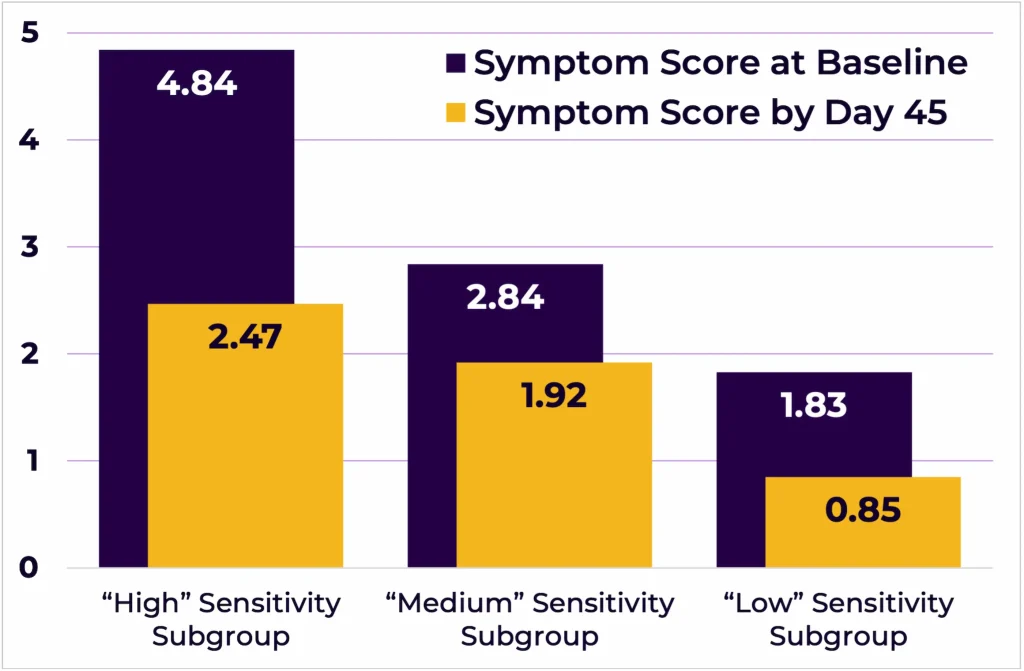
Participants were also assigned to three groups based on their sensitivity levels (i.e., subgroups – “High” n=13, “Medium” n=14, and “Low” n=13, Figure 6). Participants in the group reporting the highest symptom scores (i.e., “High”) were also the ones who improved the most (i.e., the symptom scores changed from 4.84/10 at Day 0 to 2.47/10 at Day 45 (F(2,37) = 6.459, p < 0.005)).
Limitations
There was a lack of blinding to the presence of the FLFE service in this experiment, which may have resulted in a degree of a placebo effect. This, however, was intentional to ensure thorough completion of Phase 1, as per FLFE’s Research Gold Standard (https://www.flfe.net/research/). Another limitation to this study may have been the self-selection and self-identification process of the EMF-sensitive individuals to participate in the study.
Conclusion and Future Directions
The largest EMF mitigation effects were observed not only for the symptoms that were rated as the most severe, but also in the subgroup of individuals that reported the highest sensitivities. Findings from this study replicate the previous two FLFE EMF mitigation studies and both replicate and extend FLFE customers’ spontaneous reports that FLFE can help mitigate symptoms of EMF sensitivity. Overall, the primary EMF mitigations include symptom reductions in insomnia, anxiety, depression, brain fog, pain, and flu-like symptoms. These symptoms also are typically among the most intensely and frequently reported by high EMF sensitive persons. Though not presented in this article, the EMF symptom mitigations associated with FLFE replicate across subgroups of age and sex.
Future research is planned to include a Phase 2 comparison experiment with volunteers who will be blinded (i.e., will be unaware whether they are receiving the FLFE mitigation protocol) during a specific period. In addition, future Phase 1 FLFE EMF mitigation experiments may investigate how these effects occur, including (a) exploring changes to the electromagnetic fields that may explain the EMF mitigation effects observed, and b) possible biophysical, biochemical, cellular, and physiological mechanisms in humans and other organisms (including animals and plants). In addition, FLFE plans to investigate further FLFE service development, including future upgrades to the EMF mitigating service, to enhance the emerging FLFE EMF mitigation effects.
Acknowledgements
We wish to thank Lewis Humphreys, M.Sc. and Maria Colomy, B.A. for their assistance in the early phases of this research.
References
[1] World Health Organization. (2004). Electromagnetic hypersensitivity: Proceedings, International Workshop on Electromagnetic Field Hypersensitivity, Prague, Czech Republic, October 25-27, 2004. https://www.who.int/publications-detail-redirect/9789241594127
[2] Stein, Y., & Udasin, I. G. (2020). Electromagnetic hypersensitivity (EHS, microwave syndrome) – Review of mechanisms. Environmental Research, 186, 109445. https://doi.org/10.1016/j.envres.2020.109445
[3] Nasim, I., & Kim, S. (2019). Mitigation of human EMF exposure in downlink of 5G. Annals of Telecommunications, 74(1), 45–52. https://doi.org/10.1007/s12243-018-0696-6
[4] Carpenter, D. O. (2015). The microwave syndrome or electro-hypersensitivity: Historical background. Reviews on Environmental Health, 30(4), 217–222. https://doi.org/10.1515/reveh-2015-0016
[5] Pall, M. L. (2016). Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. Journal of Chemical Neuroanatomy, 75, 43–51. https://doi.org/10.1016/j.jchemneu.2015.08.001
[6] Urbinello, D., Joseph, W., Verloock, L., Martens, L., & Röösli, M. (2014). Temporal trends of radio-frequency electromagnetic field (RF-EMF) exposure in everyday environments across European cities. Environmental Research, 134, 134–142. https://doi.org/10.1016/j.envres.2014.07.003
[7] Santos, H. L. dos, Vaca-Rubio, C. J., Kotaba, R., Song, Y., Abrão, T., & Popovski, P. (2023). EMF Exposure Mitigation in RIS-Assisted Multi-Beam Communications (arXiv:2305.05229). arXiv. https://doi.org/10.48550/arXiv.2305.05229
[8] Schwartz, G. E. (2021). Extraordinary claims require extraordinary evidence: The science and ethics of truth seeking and truth abuse. Cardiff, CA: Waterside Productions.
Appendix A
Baseline Survey for the “EMF Sensitives Phase 1 Replication Experiment with FLFE Service” Study
Participant ID
Please ensure the accuracy of your entered Participant ID. It needs to look exactly as it was sent to you.
_________________________
Permission Request
In this initial survey, we will be asking you first for your permission to make use of the responses you provide during this study.
Note: your personally identifiable information (name, address, phone number, etc.) will remain secure with the FLFE’s research project database, will not be shared with anyone, and will never be sold. You will receive the FLFE’s EMF Mitigating service free of charge for six months following the conclusion of the study, when your personally identifiable information will be utilized to create your free account.
Do you give us permission, while maintaining your anonymity, to incorporate your answers and comments you provide in this and the remaining surveys into a research database to be used for our scientific analysis?
(This information may eventually be presented, as anonymous data only, for peer-reviewed publication in a scientific journal and/or other public health venues and/or on the FLFE website.)
Yes—No
Do you give us permission to quote your comments anonymously in similar contexts (e.g., journal publication, public health venues, FLFE website)?
Yes—No
Assessment of Your Electromagnetic Sensitivity
Have you been diagnosed medically in any way as having Electromagnetic Hypersensitivity Syndrome (EHS) or Electromagnetic Sensitivity (EMS)?
Yes—No—Other
Do you currently have or have you been diagnosed in the past as having Multiple Chemical Sensitivity (MCS)?
(We ask this because scientific research has revealed a strong link between MCS and EHS/EMS.)
Yes—No—Other
Adverse Effects Rating
Please rank on a scale of 0 to 10 the degree to which you experience adverse effects from each of the following devices, where:
- 0 (zero) indicates that you do NOT experience adverse effects from it;
- 1 indicates that you do not know or are not sure – have never noticed or paid attention well enough to say for certain; or you’ve never (knowingly) been exposed to it to know;
- 2 indicates you notice slight effects from exposure to it;
- 5 indicates moderately noticeable effects – you can definitely tell it’s affecting you;
- 10 indicates severe/debilitating adverse reaction/effects to exposure to it.
Do not feel locked into integer values. You may use non-integer values (e.g., 5.3 or 7.5, etc.) if that helps you to better quantify your experience.
Effects of Device 1 – Cell Phones
_________________________
Effects of Device 2 – Cell Phone Towers
_________________________
Effects of Device 3 – Cordless Phones / Cordless Phone Bases
_________________________
Effects of Device 4 – Corded Phones with Call Waiting / Caller ID
_________________________
Effects of Device 5 – Wireless Internet (WiFi, WLAN, UMTS, WiMax)
_________________________
Effects of Device 6 – Wireless Computer Routers or Modems (also WiFi)
_________________________
Effects of Device 7 – Bluetooth Devices (Hearing Aids, Ear Buds, Speakers)
_________________________
Effects of Device 8 – Computers (including iPads / Tablets) connected to the Internet Wirelessly
_________________________
Effects of Device 9 – Other Wireless Computer Accessories (Mouse, Printer)
_________________________
Effects of Device 10 – Computers that have all Wireless Features turned off and are wired to the Internet via Ethernet Cables
_________________________
Effects of Device 11 – Other Wireless Devices (Baby Monitor, Smart Watch)
_________________________
Effects of Device 12 – 5G Antennas
_________________________
Effects of Device 13 – Wireless Smart Electric, Gas, or Water Meters
_________________________
Effects of Device 14 – Energy-Saving Light Bulbs
_________________________
Effects of Device 15 – Power Lines (particularly if located near Your Home)
_________________________
Effects of Device 16 – Power Supplies and Power Strips to Electronics
_________________________
Effects of Device 17 – Electric or Induction-Type Stoves
_________________________
Effects of Device 18 – Microwave Ovens
_________________________
Baseline
The rest of this survey will provide an initial baseline against which to compare what you experience once the FLFE field is activated.
We would like to assess how often you suffer from your symptoms in addition to their severity.
Pay attention to the time of the day when you fill out the survey (e.g., morning), aiming to do it at the same time of the day for all the surveys in this study.
Intensity of Symptoms
Please rank on a scale of 0 to 10 the intensity of the following 30 symptoms you have experienced over the last 45 days, some or all of which may be arising from your sensitivity to electromagnetic fields or radiation from wireless devices.
- 0 (zero) indicates that you do NOT have this symptom.
- 1 indicates a mild or minor discomfort from the symptom.
- 5 indicates moderately uncomfortable symptom – tolerable but just barely.
- 10 indicates severe discomfort from the symptom – an extremely uncomfortable, painful, and/or debilitating symptom, or potentially life-threatening.
Do not feel locked into integer values. You may use non-integer values (e.g., 5.3 or 7.5, etc.) if that helps you to better quantify your experience of a symptom.
Frequency of Symptoms
Please indicate on a scale of 0 to 10 how frequently you have experienced each of these 30 symptoms over the course of the last 45 days, some or all of which may be arising from your sensitivity to electromagnetic fields or radiation from wireless devices.
- 0 (zero) indicates that you do NOT experience this symptom.
- 1 indicates you experience the symptom infrequently, 10% of the time or less which works out to be about 3 times a month or less.
- 2.5 indicates you experience the symptom about 25% of the time, which works out to be 1-2 times per week.
- 5 indicates you experience the symptom about 50% of the time, say, 3-4 times per week.
- 10 indicates you experience this symptom 100% of the time, every hour of every day (continuous or chronic).
Do not feel locked into integer values. You may use non-integer values (e.g., 5.3 or 7.5, etc.) if that helps you to better quantify your experience of a symptom.
Intensity of Symptom 1 – Allergies / Food Sensitivities
_________________________
Frequency of Symptom 1 – Allergies / Food Sensitivities
_________________________
Intensity of Symptom 2 – Anxiety / Agitation / Irritability / Restlessness
_________________________
Frequency of Symptom 2 – Anxiety / Agitation / Irritability / Restlessness
_________________________
Intensity of Symptom 3 – “Brain Fog” / Unable to Think Clearly or Concentrate
_________________________
Frequency of Symptom 3 – “Brain Fog” / Unable to Think Clearly or Concentrate
_________________________
Intensity of Symptom 4 – Depression / Loss of Motivation (for no clear reason)
_________________________
Frequency of Symptom 4 – Depression / Loss of Motivation (for no clear reason)
_________________________
Intensity of Symptom 5 – Digestive Problems / Stomach Pain / Irritable Bowel
_________________________
Frequency of Symptom 5 – Digestive Problems / Stomach Pain / Irritable Bowel
_________________________
Intensity of Symptom 6 – Dizziness, Including Loss of Balance
_________________________
Frequency of Symptom 6 – Dizziness, Including Loss of Balance
_________________________
Intensity of Symptom 7 – Dysesthesia – Nerve Pain, Burning Sensations, Tremors, or Other Abnormal Sensations (prickling, aching, etc. often in hands and/or feet)
_________________________
Frequency of Symptom 7 – Dysesthesia – Nerve Pain, Burning Sensations, Tremors, or Other Abnormal Sensations (prickling, aching, etc. often in hands and/or feet)
_________________________
Intensity of Symptom 8 – Eye / Vision Problems, Blurred Vision
_________________________
Frequency of Symptom 8 – Eye / Vision Problems, Blurred Vision
_________________________
Intensity of Symptom 9 – Ears: Ringing, Buzzing or Tones in Ears (Tinnitus)
_________________________
Frequency of Symptom 9 – Ears: Ringing, Buzzing or Tones in Ears (Tinnitus)
_________________________
Intensity of Symptom 10 – Ear Pain and/or Hearing Loss and/or Hypersensitivity
_________________________
Frequency of Symptom 10 – Ear Pain and/or Hearing Loss and/or Hypersensitivity
_________________________
Intensity of Symptom 11 – Fatigue / Lethargy / Lack of Energy
_________________________
Frequency of Symptom 11 – Fatigue / Lethargy / Lack of Energy
_________________________
Intensity of Symptom 12 – Flu-Like Symptoms
_________________________
Frequency of Symptom 12 – Flu-Like Symptoms
_________________________
Intensity of Symptom 13 – Head: Weird Sensations of Pressure in or on the Head
_________________________
Frequency of Symptom 13 – Head: Weird Sensations of Pressure in or on the Head
_________________________
Intensity of Symptom 14 – Heart Racing, Arrhythmia, Palpitations
_________________________
Frequency of Symptom 14 – Heart Racing, Arrhythmia, Palpitations
_________________________
Intensity of Symptom 15 – Heart / Chest Pain
_________________________
Frequency of Symptom 15 – Heart / Chest Pain
_________________________
Intensity of Symptom 16 – High Blood Pressure
_________________________
Frequency of Symptom 16 – High Blood Pressure
_________________________
Intensity of Symptom 17 – Insomnia / Sleep Disturbances
_________________________
Frequency of Symptom 17 – Insomnia / Sleep Disturbances
_________________________
Intensity of Symptom 18 – Involuntary Muscle Contractions / Twitches / Cramps
_________________________
Frequency of Symptom 18 – Involuntary Muscle Contractions / Twitches / Cramps
_________________________
Intensity of Symptom 19 – Low Blood Pressure
_________________________
Frequency of Symptom 19 – Low Blood Pressure
_________________________
Intensity of Symptom 20 – Malaise, or Generally Feeling Unwell
_________________________
Frequency of Symptom 20 – Malaise, or Generally Feeling Unwell
_________________________
Intensity of Symptom 21 – Headaches (NOT Migraines)
_________________________
Frequency of Symptom 21 – Headaches (NOT Migraines)
_________________________
Intensity of Symptom 22 – Migraine Headaches
_________________________
Frequency of Symptom 22 – Migraine Headaches
_________________________
Intensity of Symptom 23 – Memory Problems
_________________________
Frequency of Symptom 23 – Memory Problems
_________________________
Intensity of Symptom 24 – Nausea
_________________________
Frequency of Symptom 24 – Nausea
_________________________
Intensity of Symptom 25 – Nosebleeds
_________________________
Frequency of Symptom 25 – Nosebleeds
_________________________
Intensity of Symptom 26 – Numbness in Hands or Feet
_________________________
Frequency of Symptom 26 – Numbness in Hands or Feet
_________________________
Intensity of Symptom 27 – Pain in Joints, Bones, Muscles, etc. Including Arthritic Pain
_________________________
Frequency of Symptom 27 – Pain in Joints, Bones, Muscles, etc. Including Arthritic Pain
_________________________
Intensity of Symptom 28 – Skin Sensations of Tingling, Burning or Itching
_________________________
Frequency of Symptom 28 – Skin Sensations of Tingling, Burning or Itching
_________________________
Intensity of Symptom 29 – Skin Rashes, Irritation, Discoloration, Dryness
_________________________
Frequency of Symptom 29 – Skin Rashes, Irritation, Discoloration, Dryness
_________________________
Intensity of Symptom 30 – Worsening of Other Medical Condition(s)
_________________________
Frequency of Symptom 30 – Worsening of Other Medical Condition(s)
_________________________
Please offer constructive criticism on this survey and/or this study thus far and what you believe could or should be done to improve it (leave blank if no suggestions for now).
If at any time you have any questions or concerns about this study, please contact our Research Coordinator at *.
________________________________________________________________________________________________________________________
________________________________________________________________________________________________________________________
________________________________________________________________________________________________________________________
Thank You and Next Steps
Thank you for taking the time to fill out this survey.
The FLFE Smarter EMF service will be activated on your property and on your phone (or personal object) within a few days. You will receive an e-mail confirming the receipt of your survey results and the date of FLFE service activation.










